OWLIVER®
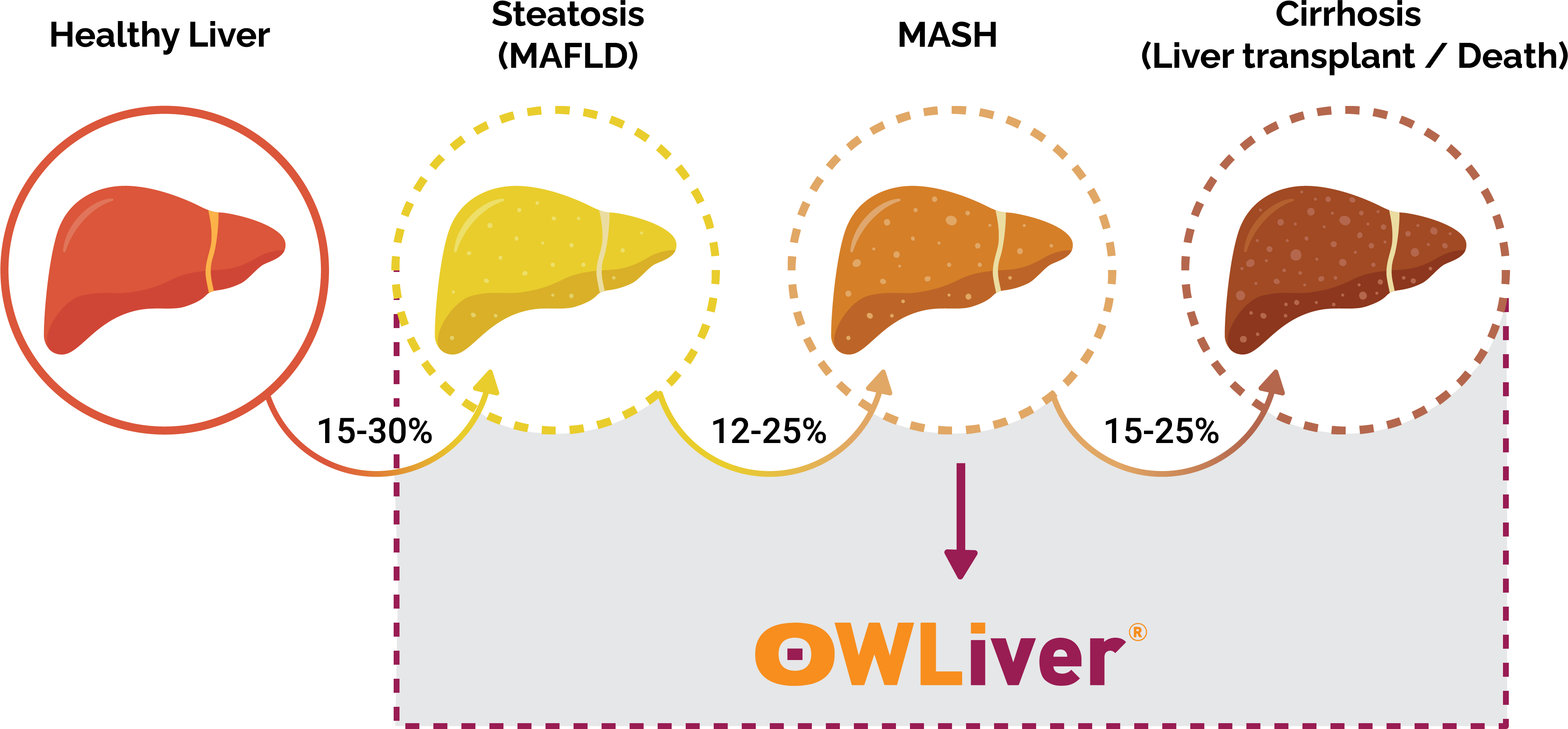
The rapid increase of obesity and metabolic syndrome is becoming a growing concern across the US as more Americans become at-risk for developing Metabolic dysfunction-Associated Steatotic Liver Disease (MASLD), formally known as Non-Alcoholic Fatty Liver Disease (NAFLD). MASLD is a type of liver disease that is not caused by alcohol and typically shows no symptoms in the early stages. As MASLD progresses, patients experience MASH (Metabolic dysfunction-Associated Steatohepatitis) which results in a dangerous toxic buildup of fat in the liver. When left undiagnosed, MASH can progress to advanced fibrosis, cirrhosis, and HCC resulting in a high rate of mortality.
Up until now, providers have had to rely on expensive imaging and invasive biopsy tests to diagnose MASLD/MASH. In partnership with CIMA Sciences, Luxor Scientific is now offering OWLiver® to the US. OWLiver® is the only non-invasive blood test available to diagnose all harmful stages of MASLD/MASH.
Using the MASEF® Score, OWLiver® is
the only non-invasive test of its kind.
OWLiver® panel includes the MASEF Score, a highly specific metabolomics-driven score developed and validated to identify “at-risk” MASH. All metabolites are measured by high performance liquid chromatography and mass spectrometry (UHPLC-MS)1,2. The analysis and determination of these metabolites reflect the amount of fat, inflammation and fiber deposits in the liver3-6. In this regard, OWLiver® makes possible to accurately establish the progression of non-alcoholic fatty liver disease (NAFLD *, or MASLD *,7). MASLD is the most common cause of chronic liver disease in the US. In fact an increase in its incidence is expected in the coming years, as it is associated with increases in obesity and metabolic syndrome8.
The relative concentrations of biomarkers are analyzed together in two algorithms that generate a final OWLiver® score. The value of the determination indicates the probability of approximation of the patient’s liver status to a healthy liver / steatosis stage, a non-alcoholic steatohepatitis (NASH *) stage, or NASH and significant-advanced fibrosis (≥F2) stage3-6.
- Specimen: Serum
- Minimum Volume: 0.5 mL
- Container: SST (serum separator tube without anticoagulant and with separating gel)
- Collection: Separate serum from cells within 45 minutes of collection via centrifugation following tube manufacturer’s instructions
- Storage: Maintain refrigerated (4°C)
- Shipping: Samples should be shipped to the laboratory on ice
- Barr J, Caballería J, Martínez-Arranz I, Domínguez-Díez A, Alonso C, Muntané J, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11(4):2521 -32.2.
- Barr J, Vázquez-Chantada M, Alonso C, Pérez-Cormenzana M, Mayo R, Galán A, et al. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated. J Proteome Res. 2010;9(9):4501 -12.
- Mayo R, Crespo J, Martínez‐Arranz I, Banales JM, Arias M, Mincholé I, et al. Metabolomic‐based noninvasive serum test to diagnose nonalcoholic steatohepatitis: Results from discovery and validation cohorts. Hepatol Commun. 2018;2(7):807 -20.



